Amniotic membrane products
iPatch™
single layer amnion, intended for ocular applications
xPatch™
single layer amnion, intended for a range of clinical applications
iPatch™ | xPatch™:
Packaged in an easy-peel, double-pouch, small form factor
Terminally sterilized to a Sterility Assurance Level of 10⁻⁶ using a validated dose of electron beam radiation
Stored at room temperature until used
Minimally manipulated during processing
Confirmed to meet all specifications prior to distribution
No preparation required
Common and custom sizes available.
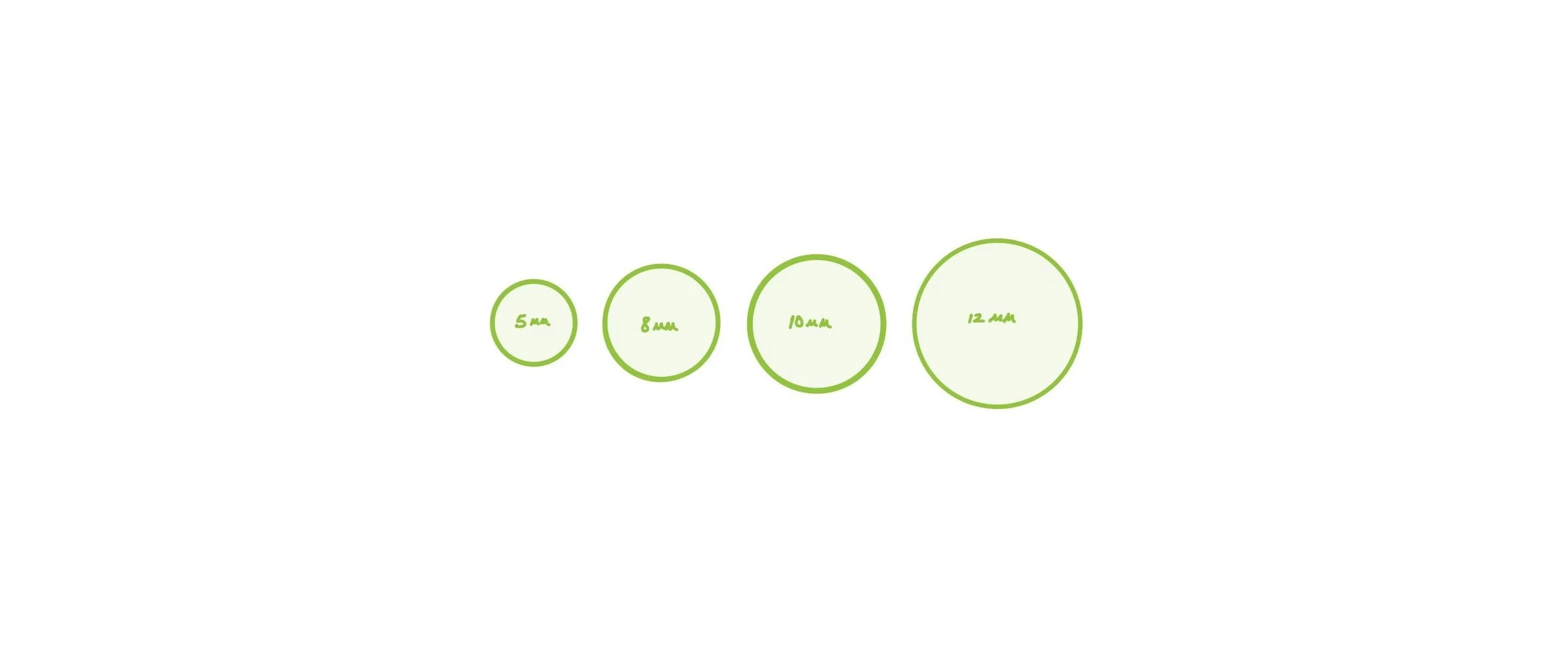
iPatch™ is designed specifically for a range of ocular indications.
Standard sizes include 5, 8, 10, and 12 millimeters (mm). Custom sizes available.
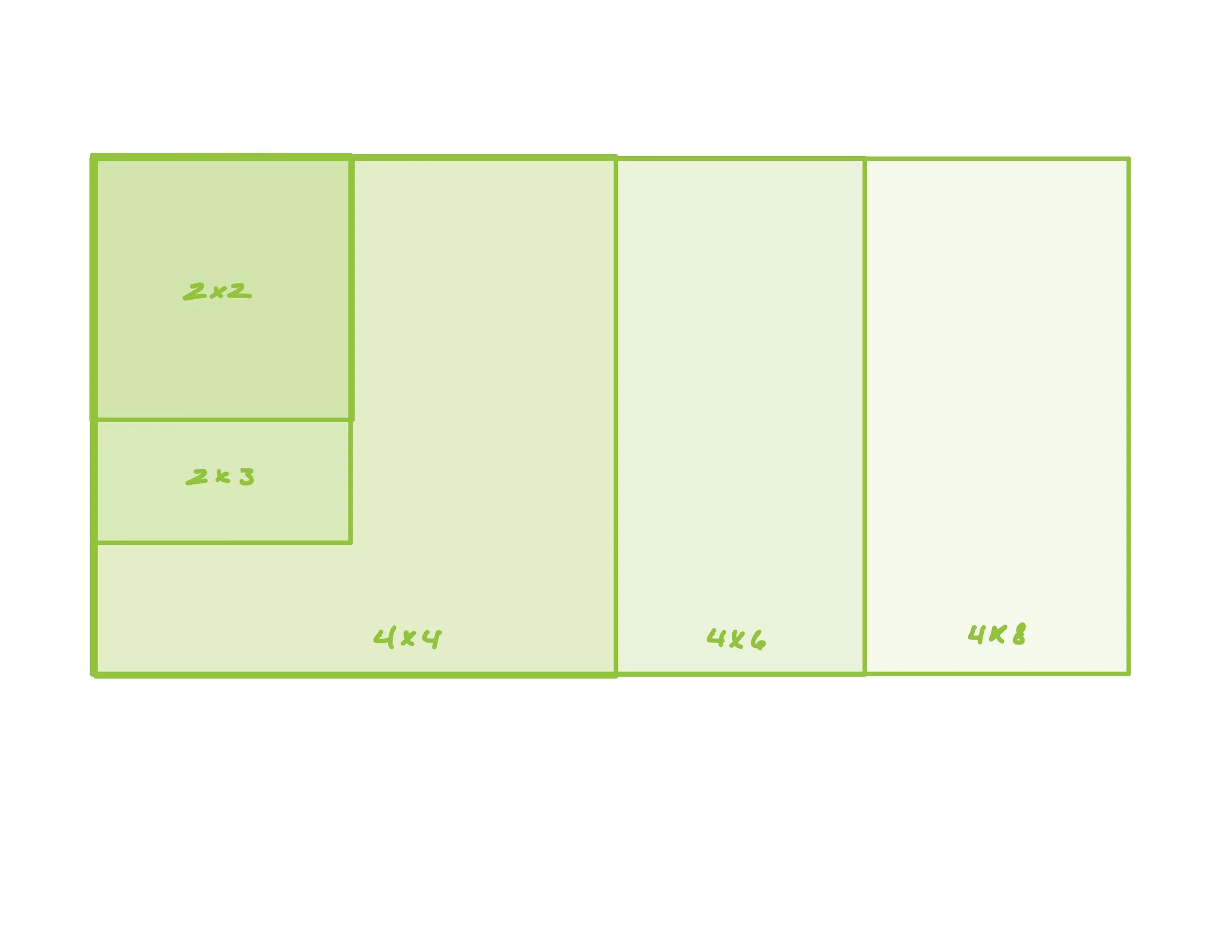
The single and double layer xPatch™ (“ex patch”) grafts are available in a wide range of standard shapes and sizes—and nearly unlimited custom sizes—to address a broad range of clinical indications. xPatch™ sizes shown in centimeters (cm).
Services
We provide branded and private label options to fit your brand and product strategy.
Our great name.
Our great products.
C5 Branded
Standard and custom sizes
Single and double layer options
Short lead time
Small form factor, user-focused C5 packaging
Your great name.
Our great products.
Private Label
Standard and custom sizes
Single and double layer options
Short lead time
Your branded packaging
Quality | Safety
Quality
C5 Biomedical’s quality management system is designed to meet FDA’s 21 CFR 1271 regulations and the American Association of Tissue Banks’ Standards for Tissue Banking. Robust manufacturing process controls ensure that all bioimplants are of the highest quality and confirmed to meet specifications prior to distribution.
Safety
All tissue donors are screened and tested in accordance with FDA’s regulations and guidance documents as well as the American Association of Tissue Banks’ Standards for Tissue Banking. Screening of donors for the following infectious diseases occurs at an FDA-registered, CLIA certified laboratory:
Hepatitis B Core Total Antibody
Hepatitis B Surface Antigen
Hepatitis C Antibody
HIV-1 & HIV-2 Antibody
HTLV I/II Antibody
Syphilis (Nontreponemal)
HIV-1/2 (NAT)
Hepatitis B (NAT)
Hepatitis C (NAT)
West Nile Virus (NAT)
Licenses | Registrations
State licenses: DE, IL, MD, OR
FDA registered